- Case report
- Open access
- Published:
A case report of pediatric acute lymphoblastic leukemia with e8a2 BCR/ABL1 fusion transcript
BMC Medical Genomics volume 15, Article number: 20 (2022)
Abstract
Background
Acute lymphoblastic leukemia is the most common type of cancer in children. Most often it affects the age group between 2 and 5 years of age. Studies have shown an improvement in general survivability, more than 90% 5-year overall survival (OS). Current treatment protocols for acute lymphoblastic leukemia require verification of the presence of favorable and unfavorable genetic abnormalities, which help qualify patients to the appropriate risk group and select a more suitable treatment. The presence of the BCR/ABL1 fusion gene stratifies the patient into a high-risk group and requires special treatment with tyrosine kinase inhibitors (TKI). The three dominant mRNA transcripts are e1a2, e13a2, and e14a2. Nevertheless, cases of atypical BCR/ABL1 transcripts have also been reported.
Case presentation
This paper presents the case of a pediatric patient with Ph + B-cell precursor acute lymphoblastic leukemia with rare atypical e8a2 BCR/ABL1 fusion transcript. Our patient achieved complete remission after 33 days of treatment. Molecular and cytogenetic studies in TP1 did not reveal the presence of the BCR/ABL1 transcript. The PCR-MRD test in TP1b was negative, the patient did not require hematopoietic stem cell transplantation.
Conclusion
Genetic evaluation of the bone marrow sample is crucial in the initial stage of the diagnosis. Fluorescent in situ hybridization and reverse transcriptase polymerase chain reaction with Sanger sequencing are the appropriate methods used in the detection of rare variants of BCR/ABL1 transcripts.
Background
Acute lymphoblastic leukemia (ALL) is the most common childhood malignancy. ALL is a heterogeneous neoplasm derived from the precursors of the lymphoid lineage. About 80–85% of cases are B-cell precursor leukemias, while T-lineage leukemias are about 15–20%. The ALL diagnoses are based on certain criteria including clinical presentation, laboratory tests, a bone marrow biopsy, immunophenotypic analysis and genetic tests. Currently, cytogenetic and molecular tests play a very important role in determining prognosis and stratification for suitable treatment of pediatric ALL [1, 2]. The typical recurrent translocations occurring in ALL are t(12;21)(p13;q22) causing ETV6/RUNX1, t(1;19)(q23;p13) causing TCF3/PBX1, t(9;22)(q34;q11.2) causing BCR/ABL1, and the most common rearrangement of KMT2A gene, t(4;11)(q21;q23) causing KMT2A/AFF1. ETV6/RUNX1 is associated with a favorable prognosis and the last three genetic abnormalities have unfavorable outcomes [3, 4].
BCR/ABL1 fusion transcripts occur approximately in 2–5% cases of childhood ALL and the frequency of BCR/ABL1(+)ALL increases with the patient’s age [5]. ALL cases with this genetic abnormality are associated with poor outcome and are qualified to the high risk group. Due to the introduction of tyrosine kinase inhibitors to the therapy, the prognosis of Ph + patients has improved.
The most common mRNA transcripts of BCR/ABL1: e1a2, e13a2, e14a2, occur in about 99% of Ph + cases. Approximately 70% of Ph + ALL patients have an e1a2 transcript and more than 25% e13a2 or e14a2. 1% of patients with Ph + shows atypical transcripts like e19a2, e6a2, e1a3, e13a3, e14a3 and e8a2 [6].
We present here a case of a pediatric patient with Ph + BCP-ALL (B cell precursor ALL) with an e8a2 BCR/ABL1 transcript.
Case presentation
An 11-year-old boy was admitted to the Unit of Pediatric Hematology and Oncology, City Hospital, Chorzów, Poland due to a suspicion of acute leukemia. Five days before admission to the hospital, he developed a severe and difficult to stop nosebleed. Since then, the boy was experienced weakness, lethargy, lack of appetite. Additionally he developed abdominal pain, a headache and nausea. Physical examination revealed pale skin with petechiae, inflammation of the gingiva, tooth decay and splenomegaly. Lymphadenopathy, hepatomegaly and the presence of a Central Nervous System (CNS) disease/leukemia were not observed. Family Health History has no indication of any genetic, hematologic or cancerous diseases. Patient was not exposed to any physical (i.e. ionic radiation) or chemical factors (organic solvents, pesticides, herbicides, paints, lacquers) during childhood nor fetal period. He was born out of second pregnancy, first childbirth (first pregnancy ended due to spontaneous miscarriage around eighth week). Weight at birth 2400 g. Mother's age at birth: 19, father: 21. Patient has younger step-sister (same mother, different father), showing no symptoms of ALL or any other hematological disorders.
By the time of diagnosis of ALL, the patient had been sick sporadically and had no routine blood tests—including morphology. The patient has not taken any medications on a permanent basis.
The laboratory results showed: white blood cell 206,900/µl, platelet count 142,000/µl and hemoglobin level 10.2 g/dl. The bone marrow was highly cellular, represented by a homogeneous population of small blasts with lymphoid morphology (88.5%). Flow cytometric analysis showed BCP-ALL phenotype: CD45dim + , CD38 + , CD34(+), CD81(+), CD24(+), CD19(+), CD79a(+), TdT(+), CD10(+), CDdim33(+), CD20dim(+), CD22dim(+), CD15(-), CD117(-). The boy was diagnosed with common B-cell precursor ALL and qualified for treatment according to the AIEOP-BFM ALL 2017 protocol.
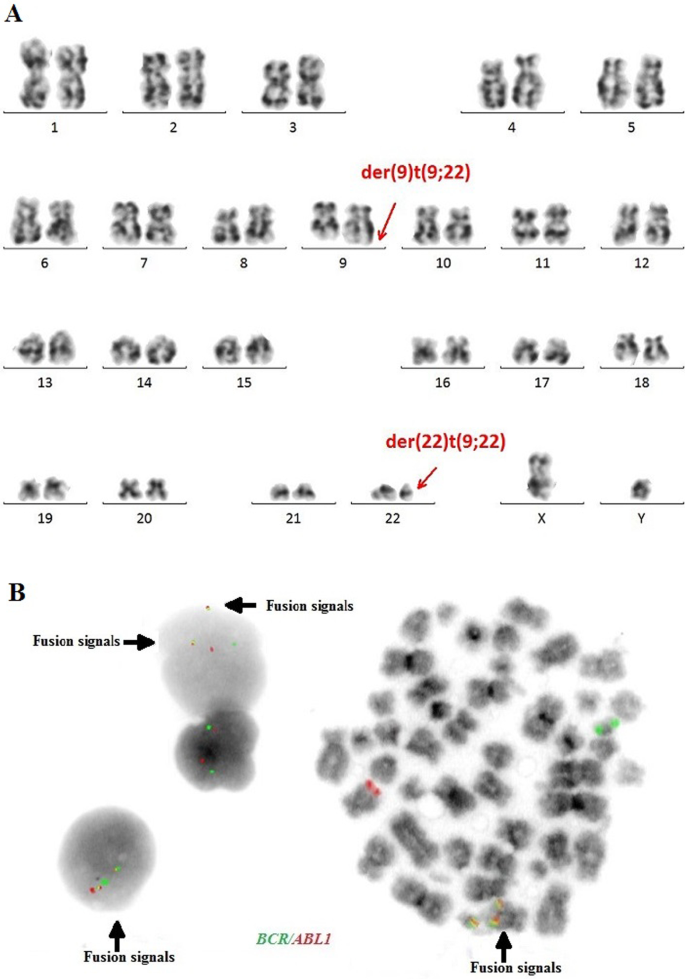
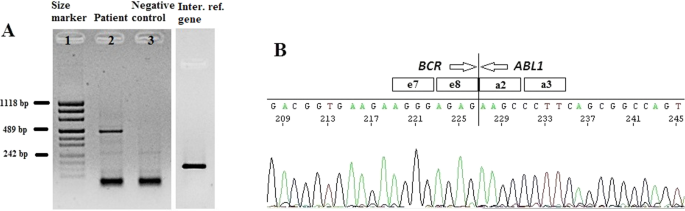
The cytogenetic and molecular examinations of the patient's bone marrow were performed by the Laboratory of Molecular Biology and Cytogenetics at the University Clinical Hospital in Wroclaw. Karyotype analysis and fluorescence in situ hybridization (FISH) was performed on the bone marrow sample according to the AIEOP-BFM ALL 2017 protocol. According to the protocol, tests for genetic diagnostics were performed. By day 6, a FISH test was performed to obtain a result for the presence of the Philadelphia chromosome. Up to day 33, the FISH test was performed for the frequent genetic aberrations: ETV6/RUNX1 translocation and rearrangements in the KMT2A and TCF3 genes. At the same time, molecular tests were carried out using the RT-PCR method for the presence of the BCR/ABL1 and KMT2A/AFF1 fusion gene. G-banded chromosome analysis revealed an abnormal male karyotype 46,XY,t(9;22)(q34;q11) [11]/46,XY [9] (Fig. 1A). The FISH study showed no rearrangements in ETV6/RUNX1, TCF3 (MetaSystems Probes, Germany) or KMT2A (Vysis, Abbott Molecular, Illinois, USA). The FISH study performed with the BCR/ABL1 dual color, dual fusion translocation probe (Vysis, Abbott Molecular, Illinois, USA) disclosed a typical translocation pattern 2 green/orange BCR/ABL1 fusion signals, 1 green BCR signal, and one orange ABL1 signal in 90% of the interphase cells (Fig. 1B). Reverse transcription-polymerase chain reaction (RT-PCR) was performed to detect the presence or absence of the KMT2A /AFF1 and BCR/ABL1 fusion gene using primers as per JJM van Dongen et al. [7]. The test was negative in both cases. Due to the positive result of the FISH test for BCR/ABL1, another RT-PCR was performed in order to search for atypical BCR/ABL1 transcripts. New RT-PCR analysis was performed based on primers BCR-6 and ABL-3 published by T. Burmeister and R. Reinhardt [6]. Electrophoresis showed a band of ~ 489 bp (Fig. 2A). Sanger sequencing confirmed the direct junction between exon 8 of BCR (NM_004327.4) and exon 2 of ABL1 (NM_005157.6) (Fig. 2B). The Sanger sequencing was important because this method determined the type of transcript by analyzing the direct junction between exons. Transcript type information is crucial for monitoring the presence of BCR/ABL1 transcript by RT-PCR method.
The patient’s induction therapy started according to the protocol IA-Pred. On the 8th day of treatment, the patient had a poor response to prednisone. Due to the presence of the BCR /ABL1 fusion gene, further treatment was performed according to the EsPhALL 2009 protocol (European intergroup study of post-induction treatment of Philadelphia-chromosome-positive ALL). Imatinib at a dose of 300 mg/m2 daily was started on day 15 of treatment, but on day 28 was withheld due to hepatotoxicity (WHO grade III). Evolution of peripheral blood cell counts during therapy is presented in Table 1. In accordance to protocol, the patient's bone marrow was collected on days 15 and 33 of treatment. Examination of the bone marrow sample on day 15 revealed 15.4% blast cells in bone marrow morphology. Flow cytometry (FCM) revealed 23.48% of blasts. On day 33 (TP1), the bone marrow was already aplastic. Nevertheless, a PCR-MRD (Minimal Residual Disease) result was obtained. MRD in TP1 was low-positive (< 10−4). Bone marrow smear revealed a total of 2.6% of blasts. Despite the poor quality of the material in TP1, it was also possible to perform a FISH study (Fig. 3A) and RT-PCR test (Fig. 3B). Both molecular and cytogenetic tests were negative. According to the EsPhALL 2009 protocol the boy should have been classified as poor risk Ph(+) ALL group because of PPR (prednisone poor responder) on the 8th day, but due to complete remission on day 33 (LBL 1.2%, PC-MRD < 10–4) he was classified as good risk Ph(+) ALL group. From about day 32 of treatment, the patient reported abdominal pain, constipation, nausea and vomiting. Physical examinations showed hepatomegaly and lazy intestinal peristalsis. The symptoms were most likely caused by paralytic intestinal obstruction after chemotherapy. Additionally, the patient developed a fungal infection of the bladder.
Due to the general condition of the patient, consolidation treatment was delayed by 25 days. After this time, according to the EsPhALL 2009 protocol the IB protocol was started and Imatinib was resumed. Another PCR-MRD test was performed on day 17 of treatment (TP1b) of the IB protocol. PCR-MRD in TP1b was negative. Therefore the patient continued chemotherapy without qualification for HSCT (Hematopoietic Stem Cell Transplantation). The patient after consolidation therapy was in haematological remission of ALL. The patient remains without a transplant for 8 months after diagnosis.
Discussion and conclusions
The very rare e8a2 transcript (about 8% from 1% of non-typical BCR/ABL1 transcripts) has been reported mainly in cases of chronic myeloid leukemia (CML) [8,9,10,11,12,13, 18]. Two cases have been reported in adult ALL [14, 15]. The e8a2 BCR/ABL1 transcript could be associated with worse prognosis than the e13a2 or the e14a2 transcripts in CML patients. However, there were cases of good response to treatment with imatinib with an achievement of a major molecular response [8, 10, 12]. CML cases with this transcript that have been reported so far, additionally had insertions from ABL1 intron 1b or 1a, from BCR intron 8 or another gene such as PRDM12, MAST2 [11, 16, 17]. Only one patient with CML and e8a2 BCR/ABL1 transcript had no additional insertions and after treatment with imatinib achieved a complete cytogenetic response [12]. In adult acute lymphoblastic leukemia one case was reported with insertion of 2 nucleotides from ABL1 intron 1a [14]. One adult ALL woman that had RALGPS1 exon 8 inserted into the fusion, was treated with FLAG-Ida (fludarabine, cytarabine, granulocyte-colony stimulating factor [G-CSF], idarubicin) and dasatinib and after re-induction therapy achieved hematological, cytogenetic and molecular remission [15]. Unfortunately, the e8a2 variant in adult ALL patients is so rare, that its impact on outcome remains unknown. To the best of our knowledge, our patient is the first pediatric ALL case with e8a2 BCR/ABL1 transcript. Our case sequencing analysis revealed e8a2 BCR/ABL1 transcript without any insertion. Creation of the e8a2 transcript by the exact fusion of BCR exon e8 to ABL1 exon a2 could encode an oncogenic protein, therefore our patient was qualified for treatment with the EsPhALL 2009 protocol. Our patient achieved complete remission after 33 days of treatment. Molecular and cytogenetic studies in TP1 did not reveal the presence of the BCR/ABL1 transcript. The PCR-MRD test in TP1b was negative, the patient did not require hematopoietic stem cell transplantation.
The presence of the BCR/ABL1 fusion gene is considered an unfavorable genetic abnormality and is associated with poor prognosis but survival has improved with the development of TKI. Our case shows that atypical transcripts of BCR/ABL1 also occur in cases other than CML or adult ALL. RT-PCR and sequencing are appropriate methods for identifying these atypical transcripts. Using both conventional cytogenetics and molecular methods, we are able to detect many genetic changes occurring in leukemias. It is important to identify them accurately and use this information to monitor the patient’s treatments. The monitoring of the presence and quantity of the BCR/ABL1 transcript using the RT-qPCR method is a gold standard in monitoring of Ph + patients with chronic myeloid leukemia. This method can also be used in monitoring of Ph + ALL patients to assess treatment efficiency. For proper patient monitoring it is important to evaluate the type of transcript at the time of diagnosis. Detection of a rare atypical transcript may affect the patient's treatment and may be associated with a worse prognosis.
Availability of data and materials
The Sanger Sequencing data generated in the study has been submitted to NCBI GenBank BankIt with the accession number OL672741; https://www.ncbi.nlm.nih.gov/nuccore/OL672741. Reference sequences used in this study are available in the following link: https://www.ncbi.nlm.nih.gov/nuccore/NM_004327.4; https://www.ncbi.nlm.nih.gov/nuccore/NM_005157.6. https://www.ncbi.nlm.nih.gov/nuccore/MF925339.1/.
Abbreviations
- OS:
-
Overall survival
- TKI:
-
Tyrosine kinase inhibitors
- BCP-ALL:
-
B cell precursor Acute Lymphoblastic Leukemia
- CNS:
-
Central Nervous System
- FISH:
-
Fluorescence in situ hybridization
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- HSCT:
-
Hematopoietic Stem Cell Transplantation
- CML:
-
Chronic myeloid leukemia
- G-CSF:
-
Granulocyte-colony stimulating factor
References
Tasian S, Loh M, Hunger S. Childhood acute lymphoblastic leukemia: integrating genomics into therapy. Cancer. 2015;121(20):3577–90. https://doi.org/10.1002/cncr.29573.
Moorman A. The clinical relevance of chromosomal and genomic abnormalities in B-cell precursor acute lymphoblastic leukaemia. Blood Rev. 2012;26(3):123–35. https://doi.org/10.1016/j.blre.2012.01.001.
Terwilliger T, Abdul-Hay M. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. 2017;7(6): e577. https://doi.org/10.1038/bcj.2017.53.
Inaba H, Mullighan CG. Pediatric acute lymphoblastic leukemia. Haematologica. 2020;105(11):2524–39. https://doi.org/10.3324/haematol.2020.247031.
Mohseni M, Uludag H, Brandwein JM. Advances in biology of acute lymphoblastic leukemia (ALL) and therapeutic implications. Am J Blood Res. 2018;8(4):29–56.
Burmeister T, Reinhardt R. A multiplex PCR for improved detection of typical and atypical BCR-ABL fusion transcripts. Leuk Res. 2008;32(4):579–85. https://doi.org/10.1016/j.leukres.2007.08.017.
Van Dongen JJ, Macintyre EA, Gabert JA, Delabesse E, Rossi V, Saglio G, et al. Standardized RT-PCR analysis of fusion gene transcripts from chromosome aberrations in acute leukemia for detection of minimal residual disease. Report of the BIOMED-1 Concerted Action: investigation of minimal residual disease in acute leukemia. Leukemia. 1999;13(12):1901–28. https://doi.org/10.1038/sj.leu.2401592.
Cayuela JM, Rousselot P, Nicolini F, Espinouse D, Ollagnier C, Bui-Thi MH, et al. Identification of a rare e8a2 BCR–ABL fusion gene in three novel chronic myeloid leukemia patients treated with imatinib. Leukemia. 2005;19(12):2334–6. https://doi.org/10.1038/sj.leu.2403986.
Demehri S, Paschka P, Schultheis B, Lange T, Koizumi T, Sugimoto T, et al. e8a2 BCR–ABL: more frequent than other atypical BCR–ABL variants? Leukemia. 2005;19(4):681–4. https://doi.org/10.1038/sj.leu.2403604.
Tchirkov A, Couderc JL, Perissel B, Goumy C, Regnier A, Uhrham-mer N, et al. Major molecular response to imatinib in a patient with chronic myeloid leukemia expressing a novel form of e8a2 BCR–ABL transcript. Leukemia. 2006;20(1):167–8. https://doi.org/10.1038/sj.leu.2404012.
Park IJ, Lim YA, Lee WG, Park JS, Kim HC, Lee H-J, Cho SR. A case of chronic myelogenous leukemia with e8a2 fusion transcript. Cancer Genet Cytogenet. 2008;185(2):106–8. https://doi.org/10.1016/j.cancergencyto.2008.06.001.
Jin C, Zhu X, Xiao M, Liu S, Liu X, Liu J, Xu X, et al. A novel e8a2BCR-ABL1 fusion transcript without insertion sequence in a patient with chronic myeloid leukemia. Ann Lab Med. 2018;38(2):169–71.
Branford S, Rudzki Z, Hughes TP. A novel BCR-ABL transcript (e8a2) with the insertion of an inverted sequence of ABL intron 1b in a patient with Philadelphia-positive chronic myeloid leukaemia. Br J Haematol. 2000;109(3):635–7. https://doi.org/10.1046/j.1365-2141.2000.02042.
Kim MJ, Yoon HJ, Park TS. The e8a2 fusion transcript in B lymphoblastic leukemia with BCR-ABL1 rearrangement. Korean J Hematol. 2012;47(3):161. https://doi.org/10.5045/kjh.2012.47.3.161.
McCarron SL, Kelly J, Coen N, McCabe S, Fay M, O’Dwyer M, et al. A novel e8a2 BCR-ABL1 fusion with insertion of RALGPS1 exon 8 in a patient with relapsed Philadelphia chromosome-positive acute lymphoblastic leukemia. Leuk Lymphoma. 2011;52(5):919–21. https://doi.org/10.3109/10428194.2011.555025.
Riva E, Manrique Arechavaleta G, De Almeida C, Costa V, Fernandez Del Campo M, Ifran González S, Uriarte R. A novel e8a2 BCR-ABL1 fusion with insertion of MAST2 exon 2 in a four-way translocation t (1;17;9;22) (p35;q24;q44;q11) in a patient with chronic myeloid leukemia. Leuk Lymphoma. 2016;57(1):203–5. https://doi.org/10.3109/10428194.2015.1043549.
Reid AG, Nacheva EP. A potential role for PRDM12 in the pathogenesis of chronic myeloid leukaemia with derivative chromosome 9 deletion. Leukemia. 2004;18(1):178–80. https://doi.org/10.1038/sj.leu.2403162.
Qin YZ, Jiang Q, Jiang H, Lai Y-Y, Shi H-X, Chen W-M, et al. Prevalence and outcomes of uncommon BCR/ABL1 fusion transcripts in patients with chronic myeloid leukaemia: data from a single centre. Br J Haematol. 2018;182(5):693–700. https://doi.org/10.1111/bjh.15453.
Acknowledgements
Not applicable.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
AM wrote the manuscript with support from TW and OH. BJ, AM conducted molecular genetics experiments and interpreted the Sanger sequencing data. SS, AT performed cytogenetical experiments. JU-R, SP, KM contributed to the clinical part of the study, prepared a clinical data and edited a clinical part of manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of Wroclaw Medical University, Poland (committee’s reference number: KB 716/2018). Written consent to participate was obtained from patient which served as negative control.
Consent for publication
Written informed consent was obtained from the patient’s parents for publication of this case report and any accompanying images. Written consent for publication was also obtained from patient which served as negative control.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mroczkowska, A., Jaźwiec, B., Urbańska-Rakus, J. et al. A case report of pediatric acute lymphoblastic leukemia with e8a2 BCR/ABL1 fusion transcript. BMC Med Genomics 15, 20 (2022). https://doi.org/10.1186/s12920-022-01169-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12920-022-01169-0